Evidence, Value, Access & Pricing
Evidence Strategy (HEOR)
A dedicated team of Subject-Matter Experts to build evidence, drive scientific and medical education, support value-based pricing and achieve favorable market access

Proven expertise in high-impact, high-value therapies with high unmet need across Pharma, Biotech and MedTech

Deep familiarity with first-to-market launches, especially in life-saving therapies for pediatric conditions and rare diseases

Creative and impactful evidence generation that differentiates products in crowded, competitive therapeutic areas (TAs)

Stellar track record in conducting RWE studies, successfully bringing them to publication and pull-through to support Market Access and Strategic Pricing

Clear demonstration of prior success in driving access for patients and reducing payer barriers for approval
In today’s global life sciences marketplace, the role and value of evidence is unquestionable. Novel therapies—many of which might be high-value therapeutics and/or specialty drugs—will need compelling evidence to persuade physicians to write the script and payers to provide access to label.
Pre-launch, clients need to characterize the disease landscape, identify unmet needs, create pre-launch medical education and conduct market-shaping activities.
Post-launch, therapies will need to continue to produce evidence of real-world value. Compelling evidence is especially needed in crowded, highly competitive TAs to demonstrate differential value versus other therapies.
Without high-quality evidence, commercial objectives suffer—market access can be sub-optimal, KOL advocacy can be weak and the product’s potential could be severely limited.
Trinity Evidence Strategy helps clients demonstrate the holistic value of their assets across the product lifecycle by leveraging a best-in-class methodological approach powered by technology and scientifically-driven, publication-quality primary and secondary research.
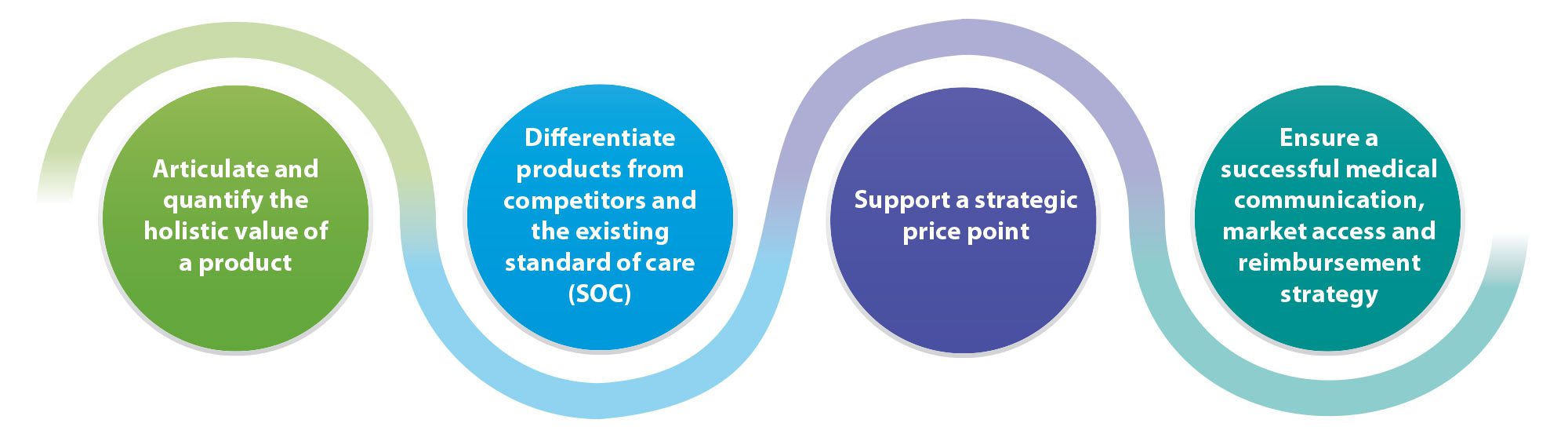
Trinity’s Evidence Strategy team offers a complete suite of HEOR and Med Affairs services to help differentiate the value of a product vs. the competition.
Evidence Planning | Evidence Generation and Execution | Scientific Dissemination
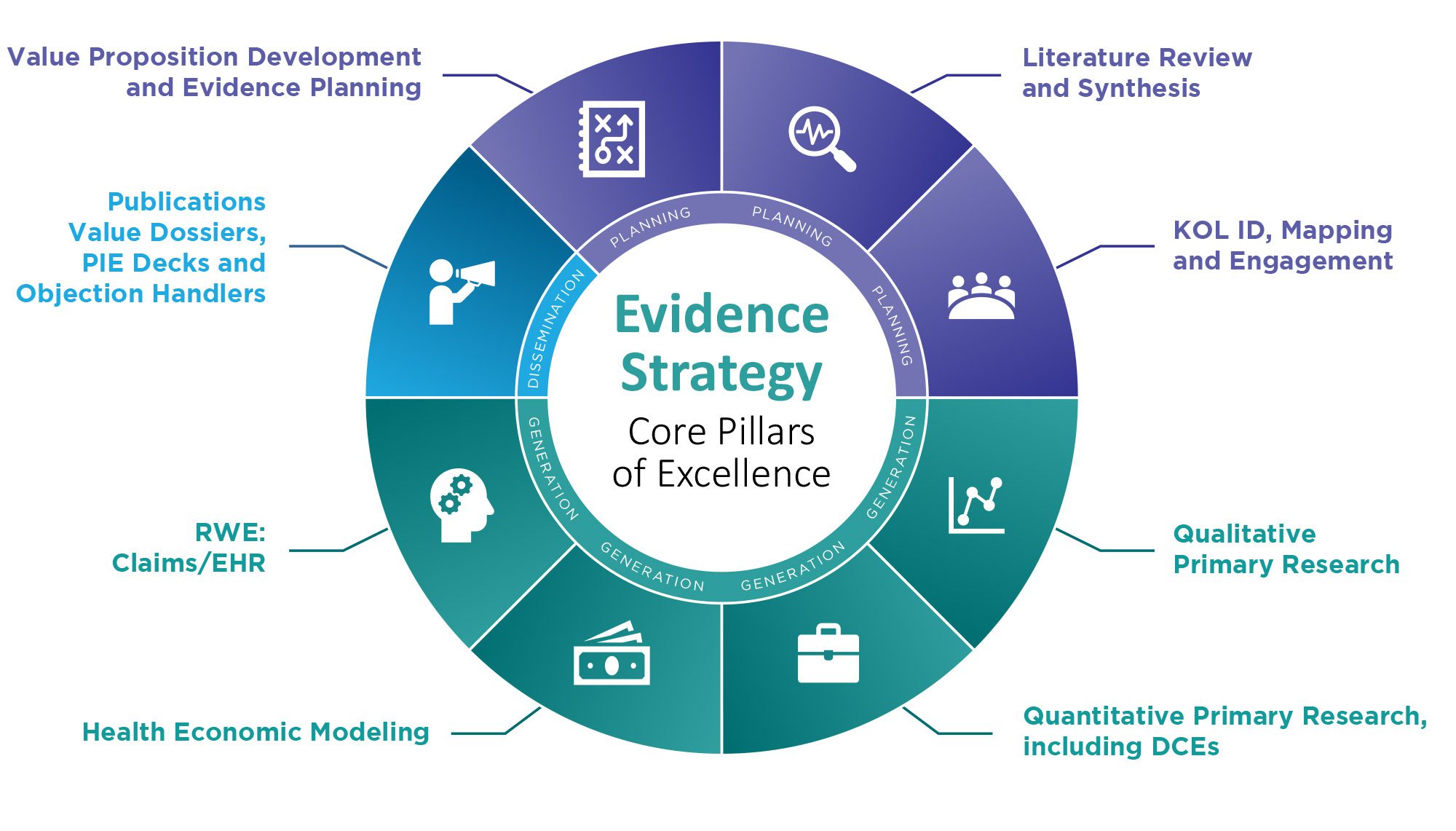
The Evidence Strategy team offers a complete suite of Health Economics and Outcomes Research (HEOR) services to help differentiate the value of a product vs. the competition.
Leveraging best-in-class, scientifically driven, publication-quality primary and secondary research methods, we help our clients demonstrate the holistic value of their assets across the product lifecycle — from early clinical development all the way to launch and post-launch.
Providing the right evidence to payers and other key access stakeholders is essential to unlock the pricing and access potential of therapies.
Find out more about Trinity’s Value services.


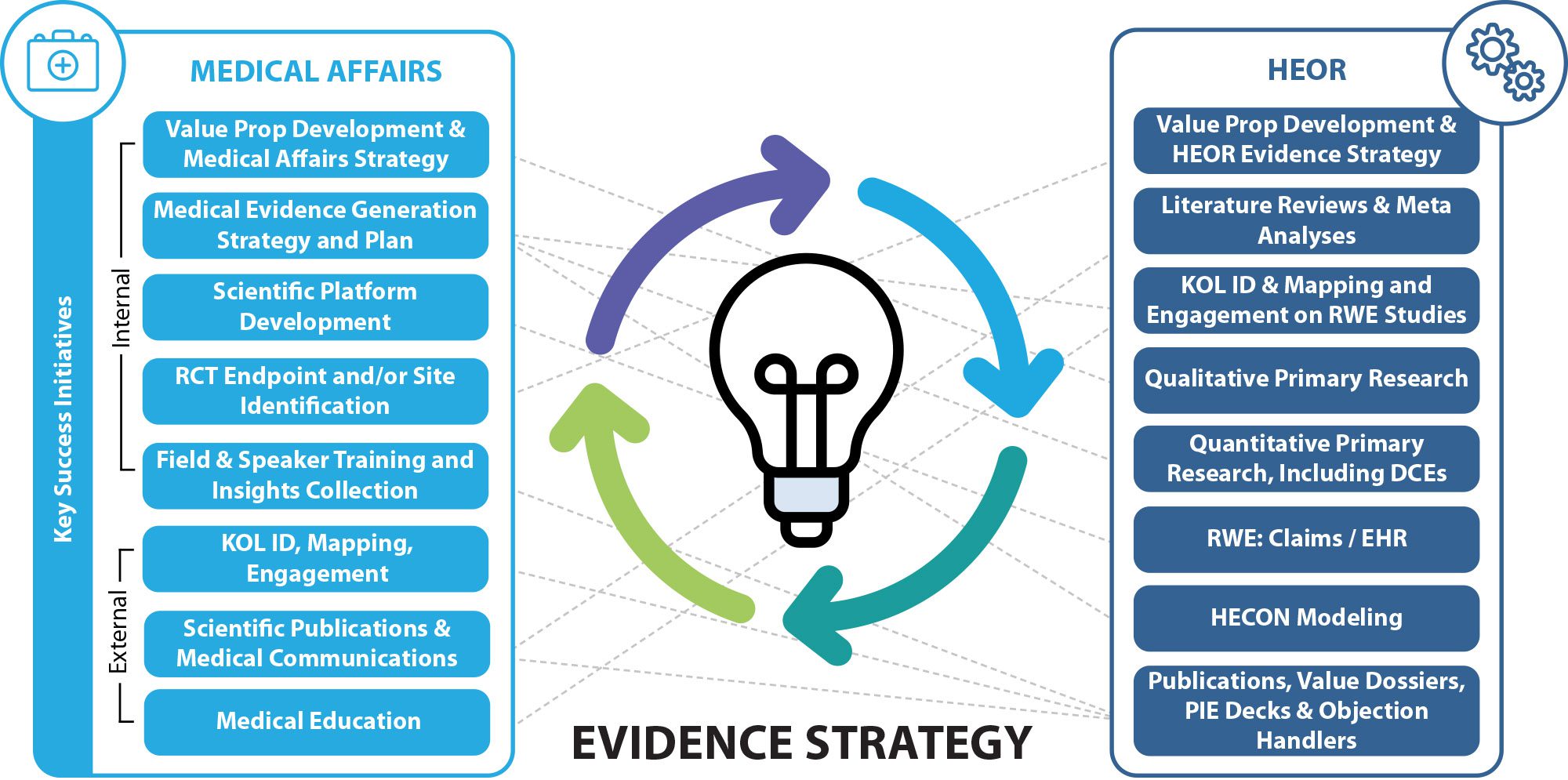
An integrated approach to evidence generation is critical to optimize strategic value stories and achieve commercialization goals. A virtuous cycle of evidence generation and value delivery is woven together by insights from randomized clinical trial and real world evidence approaches to produce poignant evidence that meets decision maker needs.
Trinity’s Evidence Strategy team achieves accuracy and confidence in identifying the right patients by using the most appropriate data assets, analytics and analytical rigor to drive the evidence needed, including Trinity’s unique network of direct data access. Trinity’s integrated, tech-enabled approach allows clients to tap into dedicated, experienced HEOR and RWE teams for support:

Which data sources have the greatest utility for each specific asset?
How—and when—
can they be
leveraged?
How should
they be combined?
What changes in data sources will influence healthcare in the future?
Trinity’s Evidence Strategy team features advanced degrees including multiple PhD-level experts – we consistently deliver high-quality insights and products to meet internal and external stakeholder needs.
The team is led by an Nandini Hadker, an Economist who presents annually at ISPOR, ISPOR-EU, AMCP, AMCP-Nexus and strategic clinical conferences who has authored numerous white papers, academic research posters and peer-reviewed manuscripts in reputed journals.
Nandini Hadker is supported by Matt O’Hara, who is also a leader in the evidence generation and HEOR space and brings deep expertise in the sphere of HEOR, evidence generation and scientific dissemination.
If you have any questions, we’re here to answer them.
We look forward to helping identify solutions for you.