The German Financial Stabilization of Statutory Health Insurance System Act
Expected Impact and Implications for Manufacturers

AMNOG* rebate negotiations will be modified to give the GKV-SV increased powers in the pricing negotiations.
HIGH P&MA IMPACT: The new negotiation framework increases the threshold of added benefit required for therapies to receive a premium vs. comparator therapies, specifically by limiting the pricing of ‘No Added Benefit’ rating products.
| HTA OUTCOME | CURRENT RULES | FUTURE PRICING RULES |
|---|---|---|
| Major & Considerable Added Benefit Rating | No Changes | No Changes |
| Minor & Unquantifiable Added Benefit Rating | Manufacturers could enter price negotiations, potentially leading to premium over branded comparator therapies | Manufacturers can only expect parity vs. a branded comparator |
| No Added Benefit Rating | Parity against comparator therapies, regardless of whether generic or branded | Guaranteed at least a 10% discount vs. non-generic comparators and at most parity vs. generic comparators |
The free pricing period for new medicines is expected to be cut from 12 to 6 months.
HIGH P&MA IMPACT: Impact revenue on the first-year post-market authorization and might see the reprioritization of Germany as a first-launch market in Europe given Germany’s inclusion in many countries’ reference baskets.

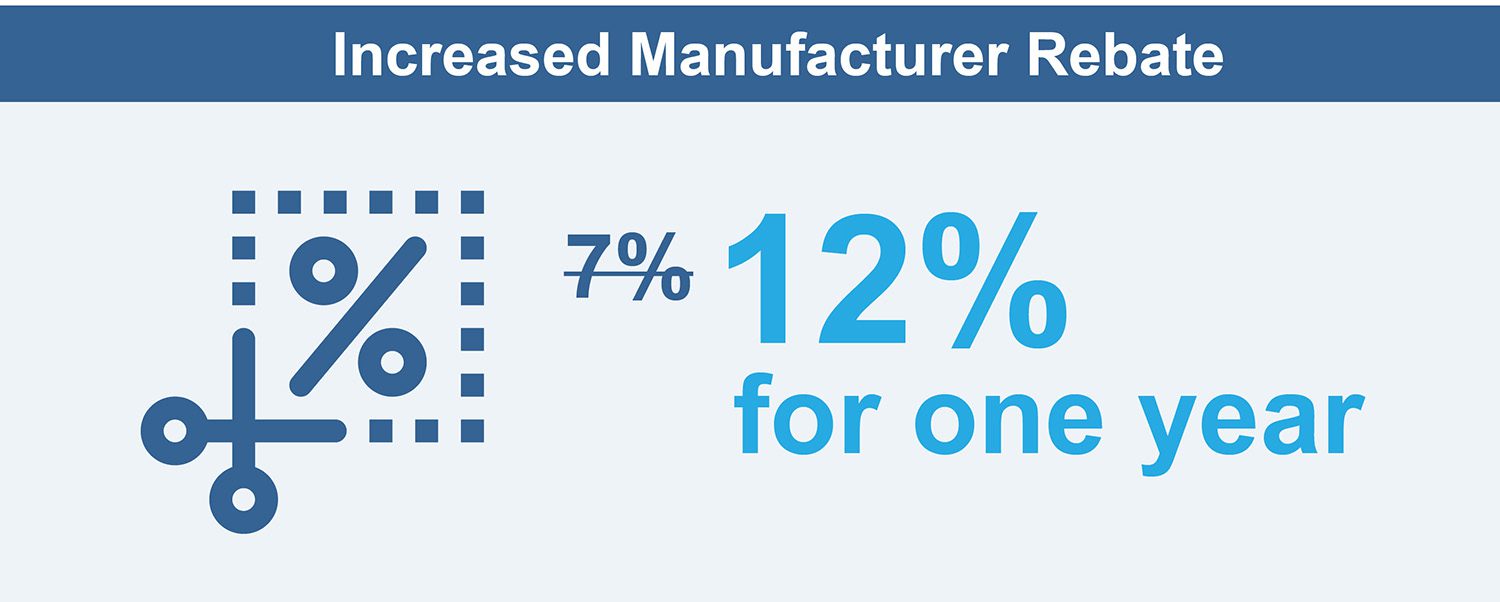
Mandatory manufacturer rebate on patent-protected drugs outside the reference pricing system will increase from 7% to 12% for one year and will be retrospectively applied on the seventh month on the market.
MEDIUM P&MA IMPACT: Manufacturers can expect to see a decrease in the price margin for drug sales with a reduction in the net-to-gross profit.
A new 20% discount will be included in the AMNOG rebate negotiation for brand-brand combination products (excluding combination therapies that receive a major or considerable added benefit rating), impacting for the first time the combination backbone which is currently not taking a price cut unless their label is updated.
HIGH P&MA IMPACT: Manufacturers can expect a decrease in the price margin for drug sales linked to a brand-brand combination, with a reduction in the net-to-gross profit.

The sales threshold at which orphan drugs become subject to the standard AMNOG evaluation process will be reduced from €50 million to €30 million per year, and only the first indication will be automatically granted additional benefit.
HIGH P&MA IMPACT: This policy will increase the number of rare disease therapies that qualify for the standard AMNOG evaluation, likely resulting in downgrades to a ‘No Added Benefit’ rating (and downstream negative pricing implications) given orphan drug evidence package limitations.

Implementation of volume-based price negotiations, new penalties for uneconomical packages and delays in the introduction of biosimilar substitution law by a year are expected in August 2023.
*The German HTA procedure for medicinal products, named after the German Medicines Market Reorganization Act in 2011
AUTHORS: Maria Florez-Cespedes, Caspar Kengeter, Salome Monreal Louly, Manon Ricard, Andreia Ribeiro
White Papers
2023 NRDL Pricing Implementation and Market Access Outcomes Update
The National Reimbursement Drug List (NRDL) negotiations are expected to conclude by November 2024, with pricing outcomes gradually being revealed starting in January 2025. The Trinity Life Sciences team has reviewed the 2023 NRDL inclusion and pricing results, drawing some key learnings.
Read More
Blog
Rise with the Waves: UK – Now and Beyond – Four Policy Trends That May Shape Pharma’s Future
Executive Summary The global payer landscape is rapidly evolving and is expected to continuously impact manufacturers’ decisions about new launches, portfolio management, trial design and importantly, pricing and market access strategy. In the UK, several policy reforms have been introduced which are expected to improve patient access while balancing financial healthcare sustainability and overall market […]
Read More
Blog
Japan Pricing Policy Reform 2024
Executive Summary The Central Social Insurance Medical Council, the key Japanese reimbursement policy panel known as Chuikyo, introduced the 2024 drug pricing reform in April 2024. Some key features of the reform plan include: The reform outlines a suite of measures aimed at driving innovation while also addressing drug lags (delay in manufacturers launching drugs in Japan […]
Read More