Home / Intelligence / Blog / Beyond the Price Tag: Understanding Colombia’s New Pricing Policy
Published November 7, 2023
In August 2023, the Colombian government published a new pricing policy outlining that the list price of new medicines in Colombia will be established based on their therapeutic value category, resulting from an assessment conducted by the Instituto de Evaluation Tecnológica en Salud (IETS). The outcomes of the therapeutic assessment are then reported to the National Commission for Prices of Medicines and Medical Devices (CNPMDM), which is responsible for setting the list price of the new drugs. Up until now, CNPMDM only set price caps, via international reference pricing (IRP), for already commercialized drugs with reduced competition or patented drugs with a declaration of public interest.
This new value-based pricing methodology seeks to increase pricing control, changing the current free pricing of new drugs after the regulator INVIMA’s marketing approval while ensuring the quality of the treatments purchased for the Colombian population to maintain the sustainability of the healthcare system. A detailed explanation of these significant changes within the new policy can be found below.

What Has Changed?
The policy has undergone several changes, including adjustments to the scope, timing, pricing methodology, rules for defining prices and the reference basket of countries. Below, a side-by-side presentation of this updated information is presented:
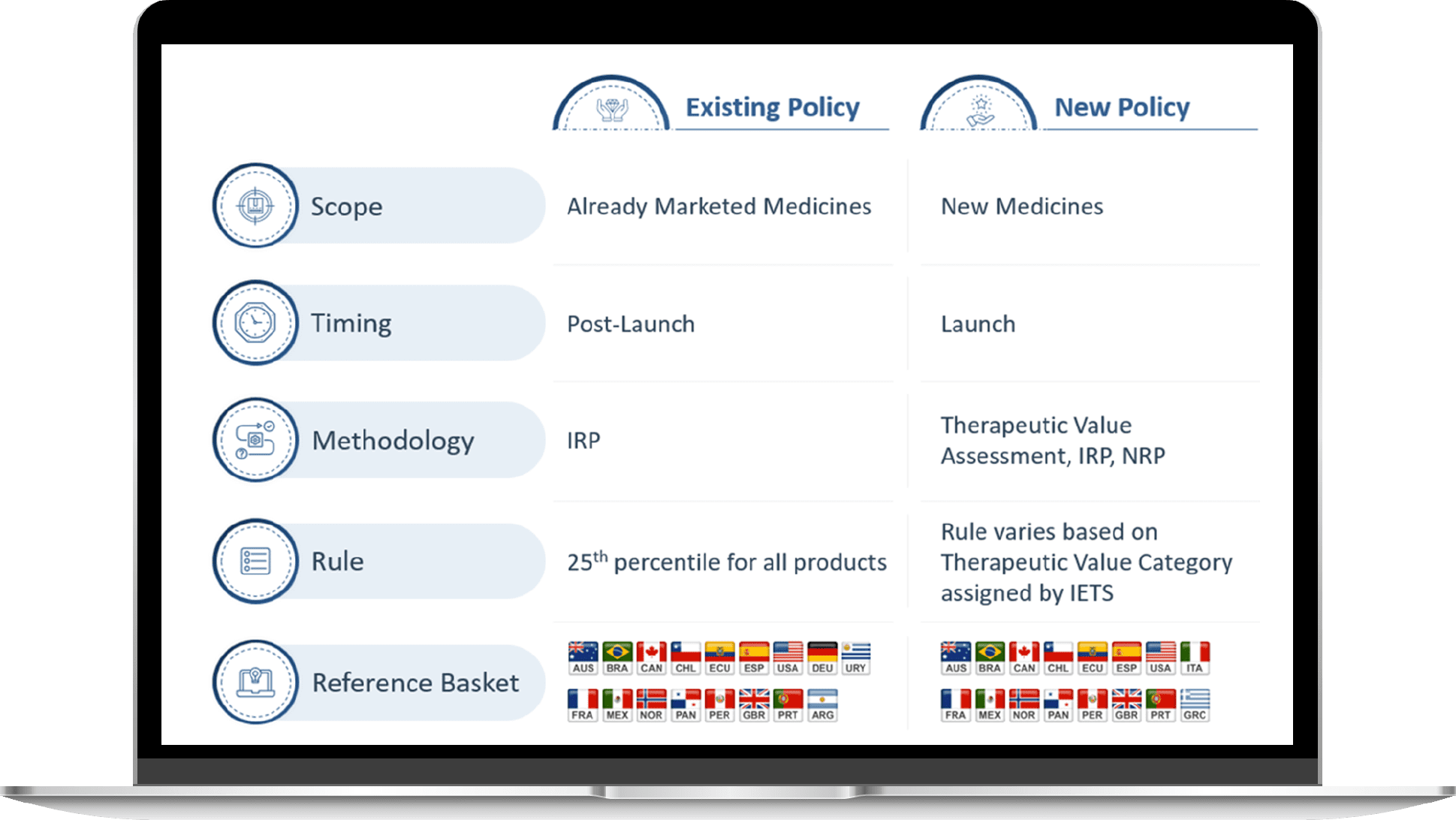
The new pricing policy will complement, not replace, the existing pricing legislation.
- Calculation of the List Price
According to the IETS evaluation, new drugs can be assigned to one of the six categories, determining each molecule’s competitiveness against the value that the new technology adds for the Colombian citizen. CNPMDM will use a different methodology to calculate the final list price depending on the therapeutic value category
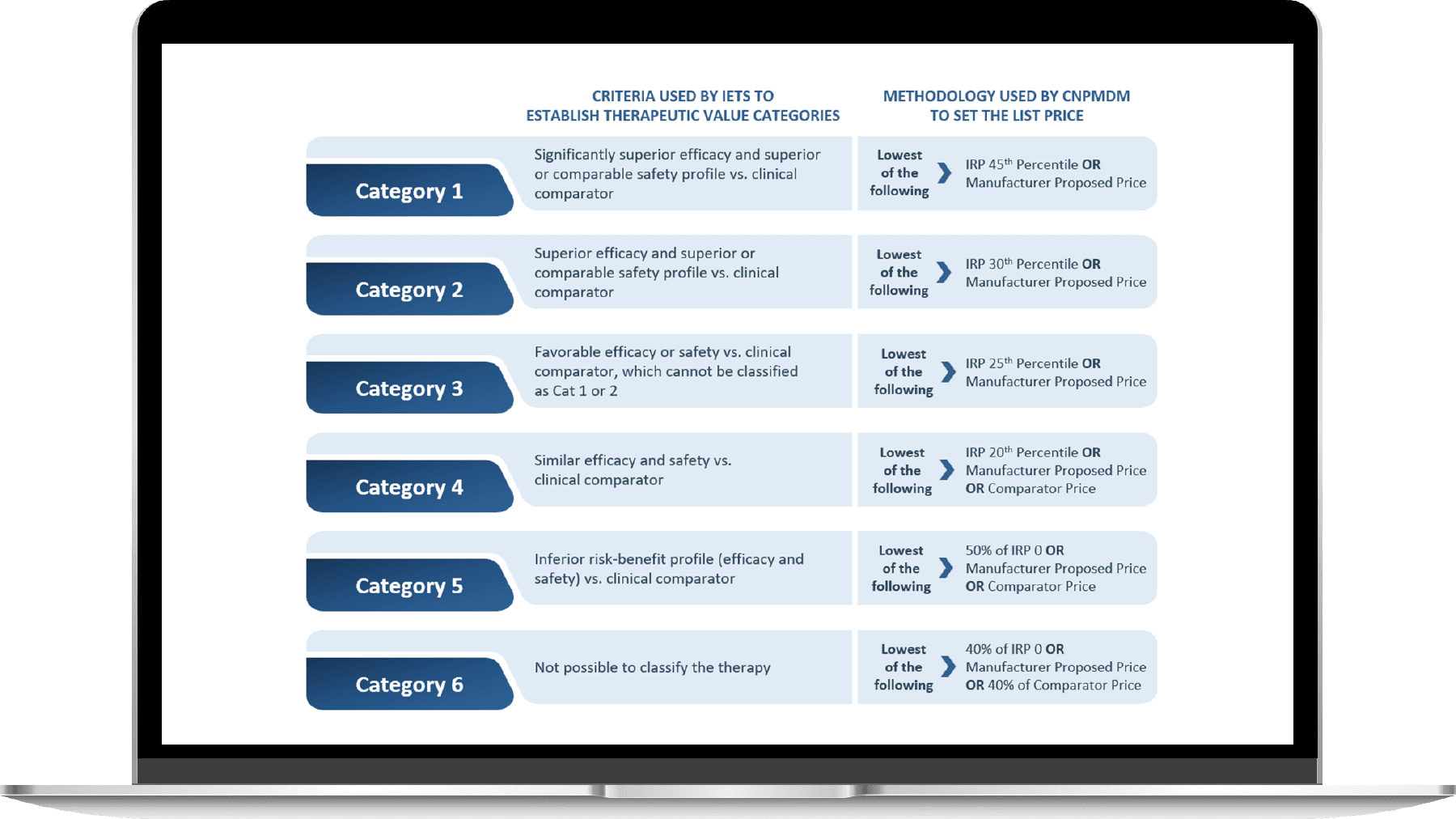
- Other considerations of this new regimen
- Multiple Indications: When more than one indication is approved for the new drug and each indication has a different category of therapeutic value, the CNPMDM will set the price according to the indication of whose category has the lowest budgetary impact.
- Validity of the List Price
- The list price for a new medicine becomes effective upon registration.
- The list price of drugs classified as CAT 1, 2 and 3 is valid for three years; after this period, CNPMDM will set the price per the current methodology for the direct price control regimen.
- Update of the List Price
- Annually, the CNPMDM will update the list price of each new drug subject to direct price control. The list price will be updated considering the value obtained in the previous point and the subsequent exchange rate changes during this period.
IRP Methodology
According to the new policy, 16 countries will be referenced by Colombia for the international reference pricing methodology. Compared to the last IRP basket, three countries (Germany, Argentina and Uruguay) were removed and replaced by Italy and Greece.
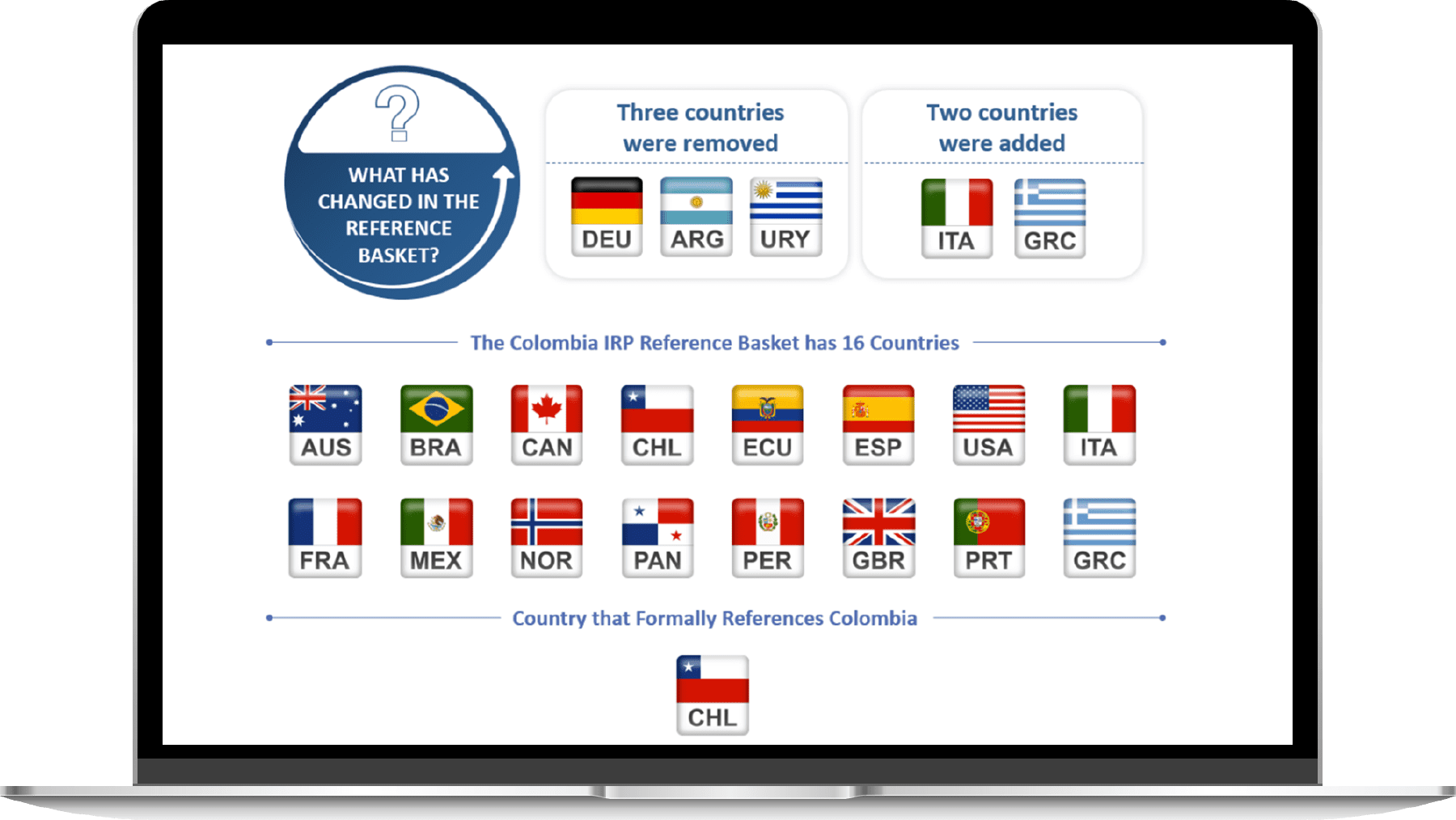
The countries composing the IRP basket were chosen based on the following criteria
- Geographical proximity
- Drug price regulation policies
- Membership in the OECD (Organisation for Economic Co-operation and Development)
- Macroeconomic stability
- Publicly available price information and data sources
The exchange rate to be used will be the nominal daily exchange rate of the currency of the reference countries expressed in Colombian pesos (COP) as published by the Bank of the Republic, corresponding to the reference period (twelve months before the date of issuance of the IETS assessment).
Additionally, there is a minimum requirement for IRP information to calculate the list price. When there is information from fewer than four reference countries, the percentile of international reference prices will not be calculated. The technical secretariat of the CNPMDM may request the manufacturer to provide additional non-confidential information on international reference prices.
- Thresholds defined by IETS to classify the magnitude of the outcomes
According to the IETS evaluation, new drugs can be assigned to one of the six categories, determining each molecule’s competitiveness against the value that the new technology adds for the Colombian citizen. Depending on the therapeutic value category, CNPMDM will use a different methodology to calculate the list price.
There will be an individual analysis for each of the selected effectiveness or efficacy and safety outcomes of new drugs by IETS. After the assessment, the magnitude of the effect will be measured against the clinical significance thresholds established in the Institute for Quality and Efficiency in Healthcare (IQWiG) guidelines. Outcomes will be classified into one of the following categories: major, considerable, minor, or no difference.
The category will be determined according to the type of outcome: binary, continuous and/or ordinal. It should be considered that thresholds will be updated if therapeutic value thresholds are available for Colombia.
- Binary Outcomes: To determine the effectiveness of the intervention, IETS will be conducting an analysis based on the relative risk (RR). A RR of less than 1 will provide evidence of a positive effect. The confidence interval (CI) of the analysis will be estimated at 95% to ensure that results are statistically significant. For time-to-event outcomes, the same table will be used to categorize and measure the effect using a hazard ratio (HR).
TABLE 1. Clinical significance thresholds to define effect magnitude in binary outcomes
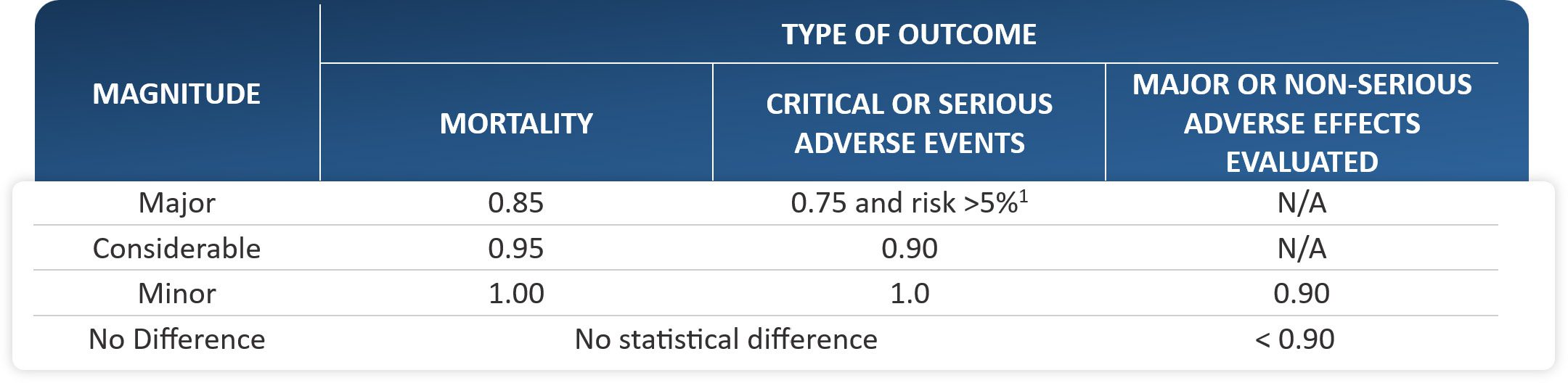
1 If the risk is not at least 5%, this category cannot be assigned.
- Continuous Outcomes: The analysis for continuous outcomes will be based on the standardized mean difference (SMD). The threshold for irrelevant results is set at SMD = 0.2 and the 95% CI must be completely above the threshold to be classified as relevant. If the benefit is a decrease in the value of SMD, the SMD and the limit values of the 95% confidence intervals will be multiplied by -1.
TABLE 2. Clinical significance thresholds to define the magnitude of the effect in continuous outcomes
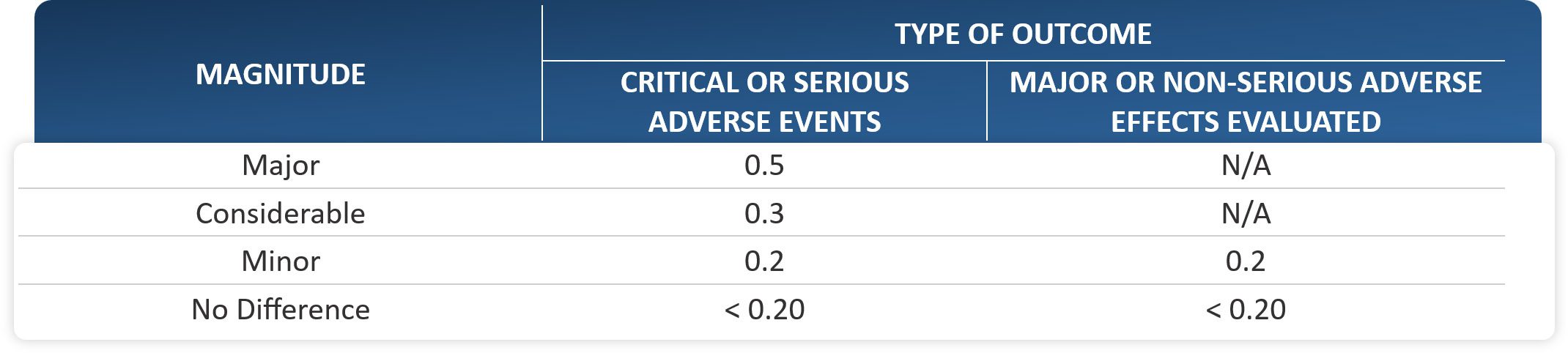
- Ordinal Outcomes: The use of the Odds Ratio (OR) derived from a multinomial logistic analysis is restricted to ordinal outcomes. To assess the effect magnitude of an ordinal outcome’s OR, the values from Table 1 will
be used
Summary Insights
Colombia has recently introduced a new pricing policy that will only apply to newly launched medicines, while existing legislation will continue to govern commercialized products.
This new policy is based on a value-based pricing approach, which means that the therapeutic value of a medicine will determine its list price. For medicines with multiple indications, each indication will be evaluated individually for value and the overall list price will be determined by the indication with the least budget impact.

Therapies that provide significant therapeutic advantages over local comparators may benefit from pricing advantages, while those with a lower GRADE of evidence or that use non-local comparators
may face stricter pricing.
This highlights the importance of payer-rationalized clinical trial design.
Download the Blog Post
References
Ministerio de Salud y Protección Social de Colombia. (2023). Circular Externa No. 16 de 2023. Originally retrieved from https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/circular-externa-16-de-2023.pdf on October 10 (no longer accessible), but available for download here.
Instituto de Estudios Técnicos en Salud (IETS). (2023). Manual Metodológico para la Definición de la Categoría de Valor Terapéutico en el Marco del Artículo 72 de la Ley 1753-2015 [PDF]. Available at: https://www.iets.org.co/wp-content/uploads/2023/04/Manual-metodologico-para-la-definicion-de-la-categoria-de-valor-terapeutico-en-el-marco-del-articulo-72-de-la-Ley-1753-2015.pdf
Authors
Lucas Tôrres, Marcela Vega, Jéssica Nacazume, Valeria Boers Trilles and Miguel Bustamante.
Related Intelligence
White Papers
2023 NRDL Pricing Implementation and Market Access Outcomes Update
The National Reimbursement Drug List (NRDL) negotiations are expected to conclude by November 2024, with pricing outcomes gradually being revealed starting in January 2025. The Trinity Life Sciences team has reviewed the 2023 NRDL inclusion and pricing results, drawing some key learnings.
Read More
Blog
Rise with the Waves: UK – Now and Beyond – Four Policy Trends That May Shape Pharma’s Future
Executive Summary The global payer landscape is rapidly evolving and is expected to continuously impact manufacturers’ decisions about new launches, portfolio management, trial design and importantly, pricing and market access strategy. In the UK, several policy reforms have been introduced which are expected to improve patient access while balancing financial healthcare sustainability and overall market […]
Read More
Blog
Japan Pricing Policy Reform 2024
Executive Summary The Central Social Insurance Medical Council, the key Japanese reimbursement policy panel known as Chuikyo, introduced the 2024 drug pricing reform in April 2024. Some key features of the reform plan include: The reform outlines a suite of measures aimed at driving innovation while also addressing drug lags (delay in manufacturers launching drugs in Japan […]
Read More



