Trinity Annual Drug Index
Evaluating the Commercial Performance and Impact of Novel Drugs Approved in 2021
This report, the seventh in our Trinity Drug Index series, outlines key themes and emerging trends in the industry as we progress towards a new world of targeted and innovative products. We provide a comprehensive evaluation of the performance of novel drugs approved by the FDA in 2021, scoring each on its commercial performance, therapeutic value and R&D investment.
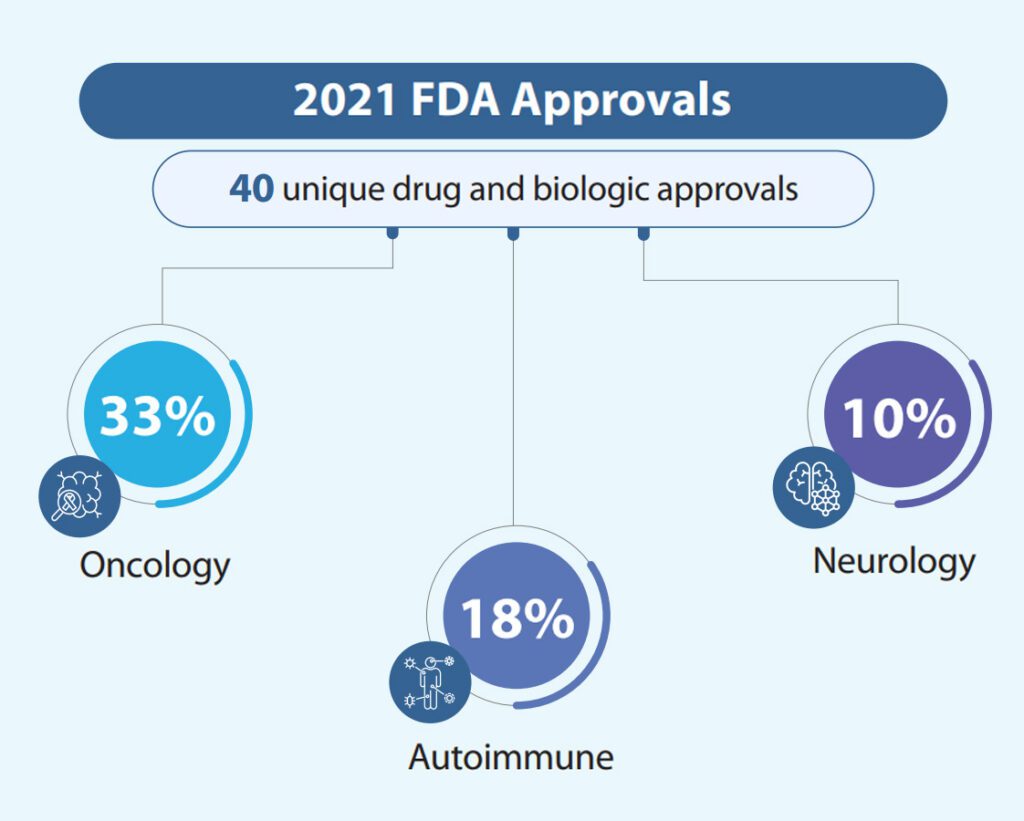
2021 saw 40 unique drug and biologic approvals, of which ~33% (13/40) were Oncology, followed by ~18% (7/40) being Autoimmune and 10% (4/40) being Neurology. In this report we describe the notable themes and trends within the industry and take a deeper look at a few products with outstanding performance. Though the impact of the COVID-19 pandemic was primarily felt in 2020, 2021 fared worse than 2020 and sustained an ongoing decline in the relationship between a product’s therapeutic value and its commercial output.
By submitting this form, creating an account, and/or using our website (or using our Services) you agree to our Privacy Policy. Information provided by you is stored in our database and may be used for sending you additional information about Trinity (including Trinity’s partners and affiliates) and our products and services. Such information may be transferred for this purpose to Trinity and affiliates in other countries. If you would like to opt out in the future, please email _compliance@trinitylifesciences.com.
This webinar will be hosted within the ON24 platform, which requires the usage of Javascript and Cookies. Please see ON24’s Privacy Policy for more information.
Blog
2025 Oncology Mid-Year Check-in: Reflections & Expectations
Reflecting on the first half of 2025 including data from AACR, ASCO and EHA, we see several notable trends continuing to shape the oncology landscape. While development follows momentum observed the last few years, these learnings provide a clearer understanding into the next wave of innovation. Immunotherapy Moves Earlier in the Journey One of the […]
Read More
Blog
Joint Clinical Assessment in the EU: What Life Sciences Companies Need to Know
March 2025 marked a pivotal moment for pharmaceutical and biotech companies operating in the European Union (EU) as the first two molecules began to proceed through the Joint Clinical Assessment (JCA) process. At a recent seminar hosted by Trinity Life Sciences, stakeholders gathered to explore the implications of this new regulatory framework and how to […]
Read More
White Papers
Predicting Winners: AI-Powered Portfolio Management
Can artificial intelligence and machine learning (AIML) be used to support portfolio decision-making across the life sciences industry? In this installment of our Industry Impact Series, we find that not only can the latest AIML techniques identify the drivers of success and failure in commercialization, but Trinity Life Sciences’ AI algorithm accurately predicts revenue outcomes for […]
Read More