Home / Intelligence / Blog / How Effective is ANVISA’s Rare Diseases Expedited Approval Pathway [RDC 205]?
Published March 18, 2022

Key Takeaways
An analysis was conducted of all the orphan therapies registered by the FDA and ANVISA before and after 2018 to effectively evaluate approval times once the RDC 205 pathway was implemented. The main takeaways from the analysis can be found below:
- The difference between FDA and ANVISA time to approval for orphan drugs is four times shorter after the implementation of the RDC 205 / 2017 pathway
- The RDC 2015 pathway regulated the deadlines for the regulatory and pricing evaluations, allowing the Brazilian government to have more control over the prices of orphan therapies
- The RDC 205 represented a greater possibility of availability of novel therapies for rare diseases for Brazilian patients
Rare Disease Landscape in Brazil ANVISA (RDC 60/2014)
In Brazil, it is estimated that 13 million people are affected by rare diseases. Rare diseases are considered a heterogenous group of conditions that typically only have limited therapeutic options available and represent a challenging unmet need as these diseases are often life-limiting and life-threatening. In these cases, accelerated approval frameworks might be critical to provide opportune access to new, safe and effective therapies to the patients.
Up until 2018, there were no specific regulatory frameworks for the approval of drugs for rare diseases in Brazil. The standard process for registering new therapies was regulated by the RDC 60/2014 and would not provide any prioritization pathway for the approval or assessment of orphan therapies. The typical approval process was through the Brazilian Health Surveillance Agency (ANVISA) and could take up to 448 days, this was from the submission phase through to approval. Since there was a lack of further incentives for manufacturers, many did not end up registering their orphan therapies in Brazil. Instead, access to orphan therapies was through importation purchases, and without locally controlled prices.
Rare Diseases Expedited Pathway ANVISA (RDC 205) Represents a Major Access Gateway to Orphan Therapies
An expedited pathway for rare disease products was finally established in 2018 with the RDC 205. This created an alternative registration avenue to encourage pharmaceutical companies with orphan therapies to register early in Brazil. The RDC 205 establishes deadlines and mechanisms to shorten the analysis timing of drugs designated for rare diseases and implemented the pre-submission meeting with ANVISA and the manufacturer. This helped to align on information about the product and relevant submission documents.
The ANVISA Expedited Pathway Process
Part of the registration process means the interested company must schedule this initial meeting within 60 days after the first submission to another regulatory agency to manifest their interest in submitting through the RDC 205 expedited pathway. With that, manufacturers are encouraged to request registration in Brazil concomitantly to other major regulatory agencies, like the Food and Drug Administration (FDA) in the United States (US), or the European Medicines Agency (EMA) in Europe. After the meeting, the request must be formalized within 30 days to ANVISA, who has 60 days to issue the final decision or request more information.
In the first case, the drug registration is published within 30 days, and, if more information is required, manufacturers have 30 days to fulfill the request, which will be assessed within 45 days by ANVISA. The analysis of the requests highlight that, after 2018, the ANVISA’s process of evaluating rare disease therapies now lasts on average 246 days from submission to approval – almost half of the previous delay time before RDC 205 was introduced.
After receiving the ANVISA marketing authorization, manufacturers must submit the pricing request to the Drug Market Regulation Chamber (CMED), the institution that regulates the maximum selling prices in the country and that will set the price at the point of registration for the first indication, which is not revisited for follow-on indications. For innovative orphan therapies with no other therapeutic options available in the market, price usually falls into Category I, being set based on lowest IRP (international reference pricing) from a defined basket of countries (United States, New Zealand, Australia, Greece, Portugal, Italy, Spain, France, and Canada). With the establishment of RDC 205, among other incentives in place, Brazil is moving up in the line of the global launch sequence of drugs intended for rare disease, allowing situations in which CMED sets a temporary maximum selling price based on the US price, which is also usually the highest price of the markets, until the drug is registered in at least other two markets.
The Benefits of ANVISA (RDC 205)
The ANIVISA RDC 205 registration pathway of drugs intended for rare diseases is faster than ANVISA’s regular procedure for other therapies. This new expedited pathway provides clear benefits for different stakeholders:
- Brazil will have new technologies entering early in the market
- The Ministry of Health and payers will have a controlled price reducing exceptional purchases that oblige them to pay high amounts of money per therapy
- Manufacturers may secure a higher price and prepare a more efficient launch strategy
- Brazilian patients will also have a greater possibility of having faster access to therapies that can help them reduce symptoms and improve their life expectancy and associated quality of life
Key Incentives for Stakeholders to Utilize ANVISA (RDC 205)
The RDC 205 expedited pathway provides different benefits and incentives for multiple stakeholders. The Ministry of Health and payers in Brazil will have the opportunity to access new therapies for rare diseases earlier and with a locally controlled price. At the same time, manufacturers may secure a higher list price for their products and are encouraged to define value, access and pricing strategies for Brazil earlier in the global launch sequence.
Patients can be treated faster and with the latest technologies available for rare diseases, in turn reducing the clinical and economic burden of these diseases today and in the future. Overall, these benefits are being felt today and will continue to make a positive impact in the future since Brazil is making new technologies available for rare diseases early in the market.
Complete the form below to access full data: 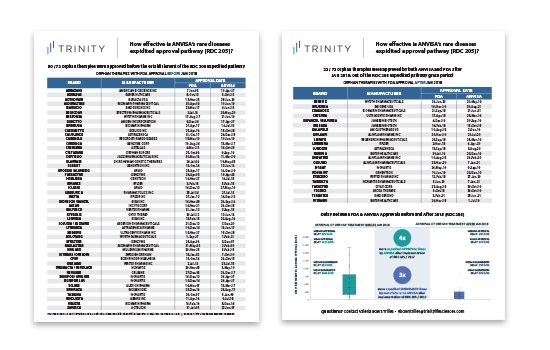
By submitting this form, creating an account, and/or using our website (or using our Services) you agree to our Privacy Policy. Information provided by you is stored in our database and may be used for sending you additional information about Trinity (including Trinity’s partners and affiliates) and our products and services. Such information may be transferred for this purpose to Trinity and affiliates in other countries. If you would like to opt out in the future, please email _compliance@trinitylifesciences.com.
Authors: Marcela Vega, Jessica Nacazume
Related Intelligence
Blog
NRDL 2024: Rare Diseases Deep Dive
China’s pharmaceutical landscape is not only vast in scale but also rapidly evolving with an emphasis on balancing access with affordability. This year’s NRDL update stands out. The introduction of the value rating system continues to raise the bar for clinical innovation, rewarding innovation that truly addresses unmet needs and demonstrates clear differentiation. The National […]
Read More
Blog
Joint Clinical Assessment in the EU: What Life Sciences Companies Need to Know
March 2025 marked a pivotal moment for pharmaceutical and biotech companies operating in the European Union (EU) as the first two molecules began to proceed through the Joint Clinical Assessment (JCA) process. At a recent seminar hosted by Trinity Life Sciences, stakeholders gathered to explore the implications of this new regulatory framework and how to […]
Read More
Blog
Pricing and Access in Germany: Innovation and Strong Evidence Rewarded
Germany’s Medical Research Act (Medizinforschungsgesetz or MFG), which came into force on October 30, 2024, is a major legislative reform aimed at strengthening Germany’s position as an attractive environment for medical innovation and pharmaceutical development. The act provides for confidential negotiated drug pricing, incentives for local clinical trials, simplified clinical trial approvals and harmonization of […]
Read More
