Practice Case Study
Case Background
Our client is a mid-size pharmaceutical company historically focused in pediatric conditions, but currently pursuing a pipeline of new medications and technologies through internal R&D and strategic industry partnerships.
Our client is seeking to explore opportunities within dermatology and ophthalmology, and has come to us for a strategic recommendation on which of these markets they should enter and how. After preliminary research and interviews with KOLs, your team has identified the following assets for potential acquisition:
Product A for Hyperhidrosis (HH)
Product B for Wet Age-related Macular Degeneration (wAMD)
Disease Background – Hyperhidrosis
Disease Overview
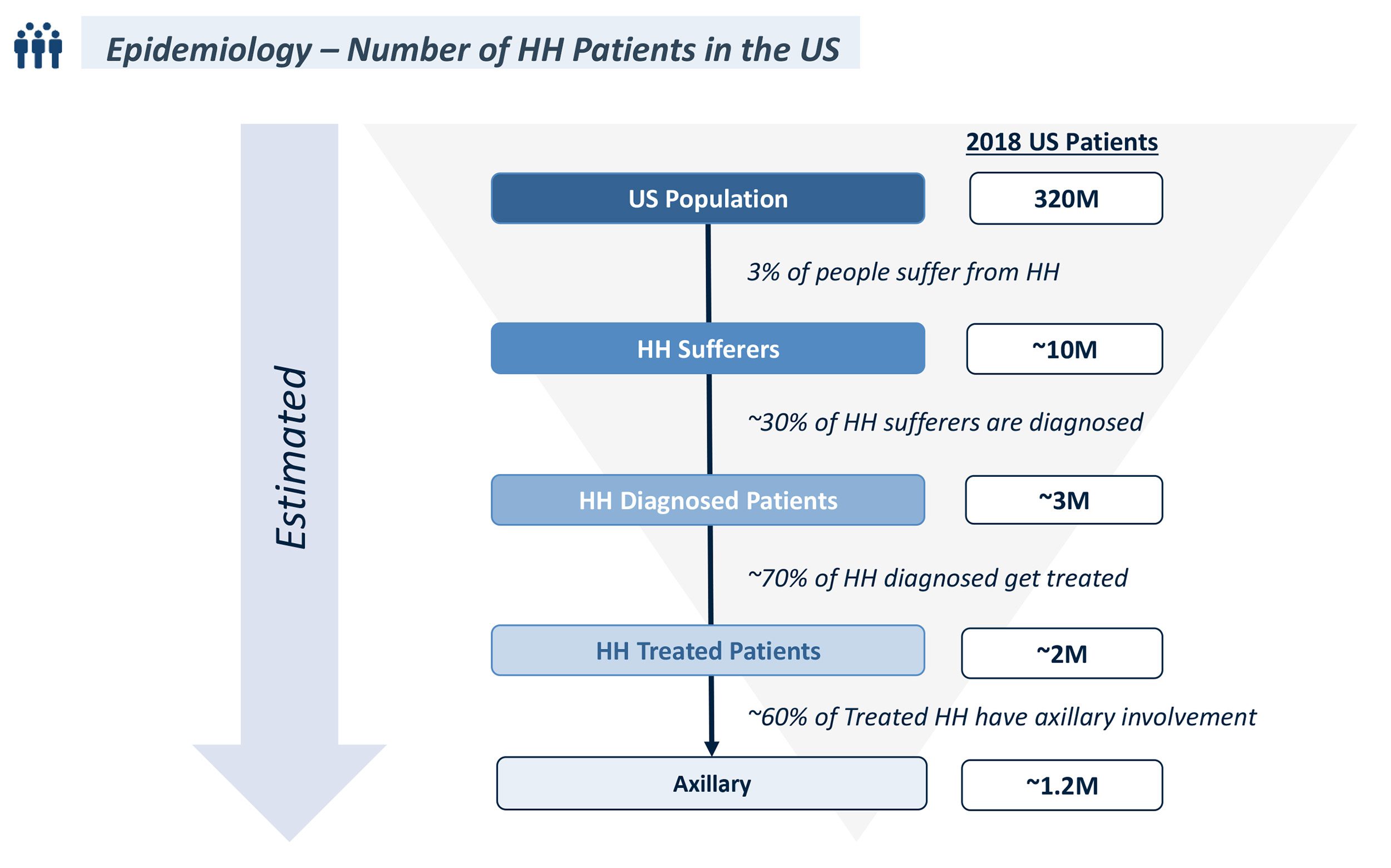
- Hyperhidrosis (HH) affects 3% of the US population and is a skin disorder characterized by sweating excessively, profusely, and visibly without exertion; ~30% of patients with HH are diagnosed
Segmentation
- Primary Focal HH (Idiopathic HH) is the most common form of HH (~90% of cases), 30-60% of patients have family history of the disease
- Secondary Generalized HH occurs due to an underlying medical condition such as diabetes or diseases involving the thyroid
Prognosis
- Although Primary HH may improve with age, it often worsens during adolescence and early adulthood
- Secondary HH can be managed by treating the underlying condition and typically has a better prognosis than Primary HH
Treatments
- 70% of all diagnosed HH patients are treated, but treatment approach varies with disease severity
- 1st line: Topical antiperspirant treatment
- 2nd line: Oral anticholinergics and/or Botox injections, microwave thermolysis to destruct sweat glands
- 3rd line (severe patients): Endoscopic thoracic sympathectomy (ETS) procedure to destruct a portion of the sympathetic nerve
Product A
- Topical anticholinergic specifically indicated for Axillary Hyperhidrosis (AHH), or excessive sweating of the underarms, which affects 60% of diagnosed, treated HH patients
- 20% improvement on all sweat measuring scales compared to SOC with limited side effects
- Currently in phase 3 of development
Disease Background – Wet AMD
Disease Overview
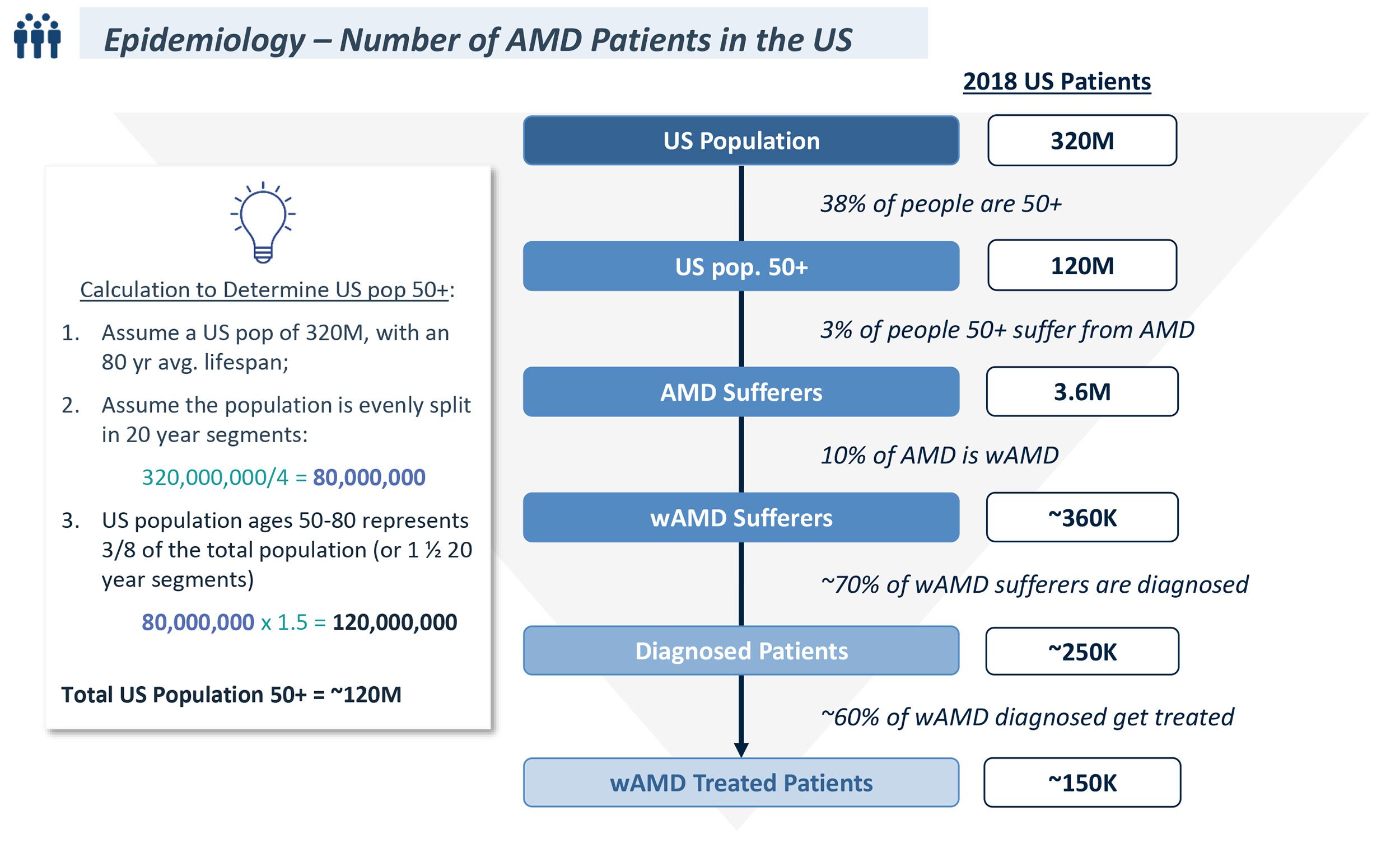
- AMD is a degenerative disease of the retina that slowly destroys the macula and may lead to visual impairment and blindness, affecting 3% of the population over the age of 50
- Wet AMD is the most severe form of age-related macular degeneration, affecting 10% of total AMD patients and 90% of those with severe vision loss caused by macular degeneration; 70% of wAMD patients are diagnosed
Prognosis
- Occurs most commonly in patients over the age of 50
- Tends to progress in the span of weeks or months, ~40% of patients develop bilateral disease
- With treatment, some wAMD patients see improvement in disease progression
Treatments
- 60% of diagnosed wAMD patients are treated, the standard of care is below:
- Intravitreal (IVT) injections: In-eye injections administered by a retina specialist on average 8 times / year
Product B
- IVT Biosimilar
- Currently in phase 3 of development
Question 1: HH Market Sizing
To initially understand the business opportunity of each indication and help prioritize our client’s entry strategy, please:
- Approximate the number of hyperhidrosis (HH) patients eligible for Product A in the United States
ANSWER:
Question 2: Wet AMD Market Sizing
Now that you’ve assessed the size of the Hyperhidrosis (HH) market, please:
- Approximate the number of Wet AMD patients eligible for Product B in the United States to compare to your previous work
ANSWER:
Question 3: Peak Revenue Potential
Now that you’ve assessed the size of the addressable patient population for Products A (Hyperhidrosis) and B (Wet AMD), the client has asked that your team evaluate and make a recommendation for which product (and indication) to pursue
As your team decides on next steps:
- Please describe the factors which would be important to consider in any recommendation for which product (and indication to pursue)
ANSWER:
- Current and evolving competitive landscape
- Market share potential based on anticipated differentiation of client’s product (e.g., efficacy vs. safety/tolerability vs. convenience)
- Pricing opportunity
- Commercial expenditure and investment requirements
- Strategic fit with company vision
Question 4: Peak Revenues Calculation
Using the information provided, please:
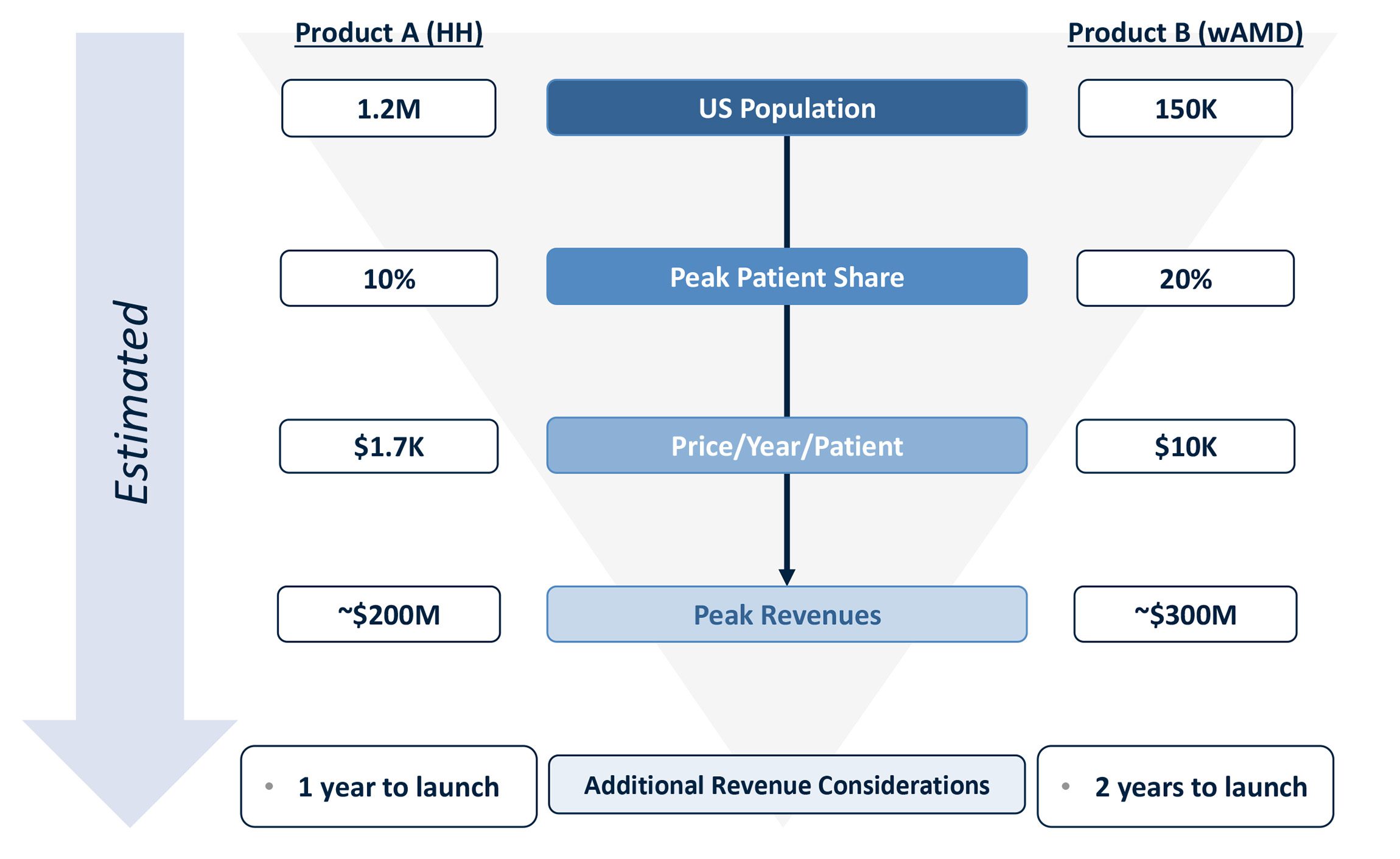
- Calculate the peak revenues of Product A (Hyperhidrosis) and B (Wet AMD)
| Product A (Hyperhidrosis) | Product B (wAMD) | |
|---|---|---|
| Addressable Population | 1.2M | 150K |
| Peak Share | 10% | 20% |
| Price/Year/Patient | $1.7K | $10K |
| Launch Year |
|
|
ANSWER:
Question 5: Indication Prioritization
Now that you have estimated the revenue opportunity associated with each product, your team has regrouped internally to brainstorm any additional considerations which could support either recommendation to the client
As you prepare for this internal working session:
- What additional considerations would support a recommendation to pursue Product A for Hyperhidrosis (HH)?
ANSWER:
While the hyperhidrosis market opportunity (i.e., axillary HH patients treated today) represents a large patient opportunity (~1.2M US patients), our client’s product is expected to capture a lower percentage (~10%) of the overall addressable population. Additionally, the pricing opportunity for Product A in Hyperhidrosis is lower than that of Product B in Wet AMD, which leads to a lower overall revenue opportunity compared to Product B ($200M vs. $300M).
That said, given HH typically worsens during adolescence vs. Wet AMD, which most commonly occurs in patients over the age of 50 years, Hyperhidrosis represents an attractive market for the client given their existing presence in pediatric conditions. As a next step, we would want to understand the commercial footprint required to support a successful launch in HH given many patients diagnosed with Hyperhidrosis are largely managed by PCPs.
Lastly, Product A is expected to generate revenue sooner, being only one year out from launch. This would help support the valuation of Product A vs. Product B, and should be considered as a part of the overall valuation.
Question 6: Indication Prioritization Cont’d.
Now that you have estimated the revenue opportunity associated with each indication, your team has regrouped internally to brainstorm any additional considerations which could support either recommendation to the client
As you prepare for this internal working session:
- What additional considerations would support a recommendation to pursue Product B for Wet AMD?
The Wet AMD market would represent more of a divergence from the company’s existing focus on pediatric conditions, but could represent an attractive opportunity, given the severity and level of unmet need for patients diagnosed with Wet AMD.
Despite a lower patient volume for Wet AMD, Product B is able to convert a larger percentage (20%) of patients to Product B. Additionally, given the severity of Wet AMD, Product B is able to achieve a much higher price point than Product A in Hyperhidrosis ($1.7k vs. $10k per patient per year). Thus, despite the smaller patient population, Product B achieves higher peak revenues than Product A ($300M vs. $200M).
While pursuing Product B in Wet AMD would be a departure from the client’s existing focus, the small (and likely concentrated) prescriber base for Wet AMD would require less investment to realize this opportunity. Since Wet AMD is treated by a specialized physician (i.e., Retina specialists), this would likely require a much smaller field force to support the commercial launch vs. HH.
Given Product B is a biosimilar in ophthalmology, pursuing Product B would likely need to be a part of a broader strategy either in biosimilars (given pricing dynamics and strategies which would need to be evaluated) or ophthalmology (given specialized prescriber base).