The Future of Cell Therapy: What’s Next?
Executive Summary
- Cell-based therapies are emerging as a promising strategy for cancer and are generating a lot of interest both academically and industrially
- To date, cell therapies have been approved in the US by the Food and Drug Administration (FDA) with them showing a lot of promise in a limited number of solid tumors although they have not yet progressed to FDA approval
- There have been multiple challenges involved when trying to successfully commercialize the products, but the landscape looks promising for the future of cancer treatment
Trinity’s Take: New cell therapy technologies show blockbuster potential for improved accessibility and efficacy in a wide range of indications, though these therapies come with their own downfalls that healthcare systems will need to adapt to in order for these products to reach their full potential
Current Cell Therapy Landscape
Cell therapies have shown exciting potential to provide a durable long-term benefit to some of the deadliest cancers. Current CAR-Ts are indicated in Acute lymphoblastic leukemia (ALL), Diffuse large B-cell lymphoma (DLBCL) and Mantle cell lymphoma (MCL), but investigational agents are being studied against a wide range of targets in multiple tumor types.
There have been several notable challenges for cell therapies, including a manufacturing bottleneck, the need to streamline and simplify the supply chain to help bring the manufacturing costs down, and an urgency to address the reimbursement gap for the treatment, particularly on the FFS Medicare side in the USA. While there are ongoing mitigation strategies in place to enable the successful commercialization of these products, what is next for cell therapies, and how will the ensuing wave of innovation present new opportunities and challenges?
A Breakdown of Cell Therapies Available
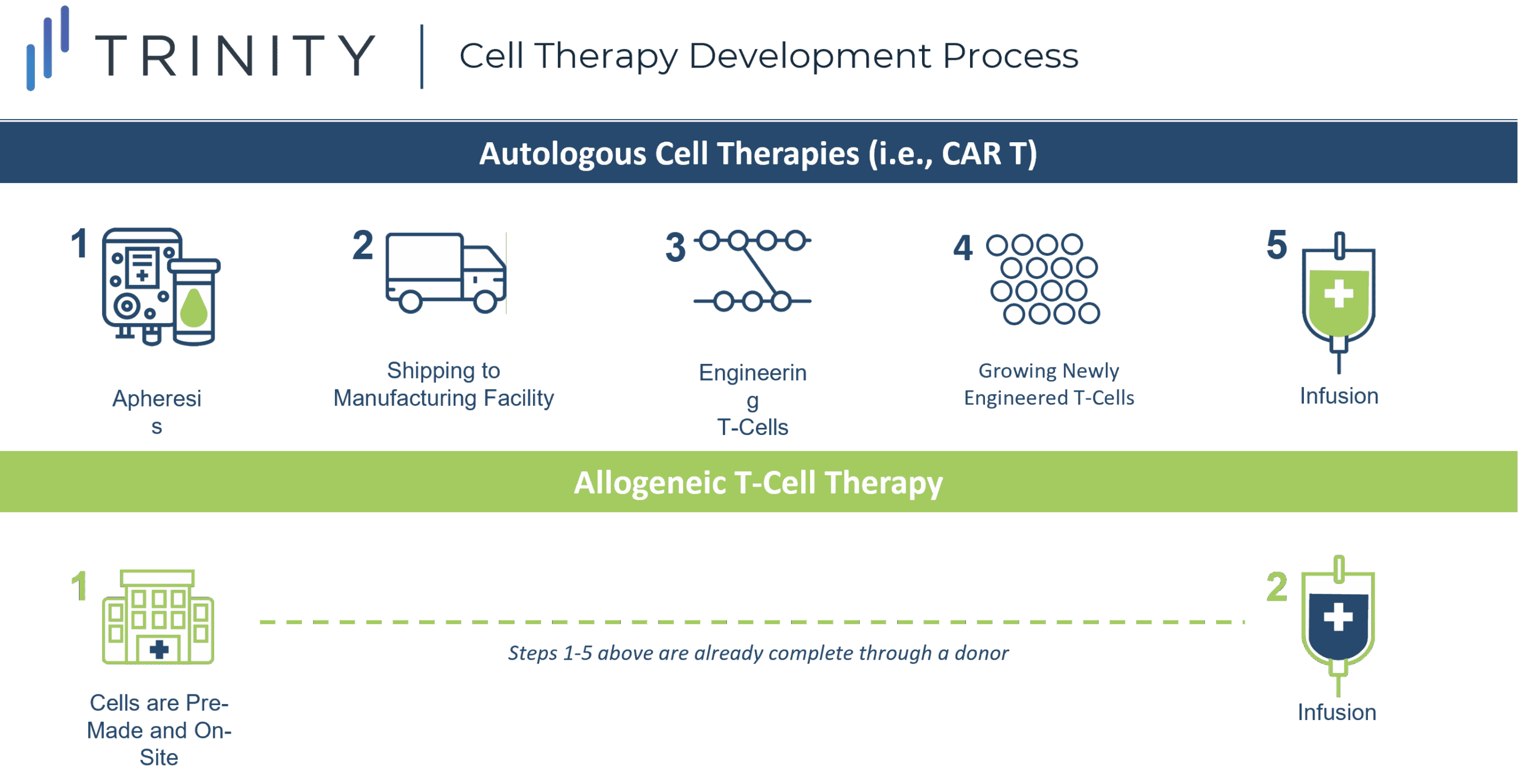
Allogeneic T cell Therapies “Off-the-shelf”
The off-the-shelf concept refers to creating CAR-T cells from a healthy donor (allogeneic), instead of harvesting and genetically modifying the T cells from the individual receiving the treatment (autologous). Autologous CAR-T therapy requires a multistep process and this increases the risk of production failure, delays, and in some cases, denied access to the therapy.
One of the most promising approaches in anticancer therapy, allogeneic T cell therapy, can be pre-manufactured for immediate use without the complexities and risks associated with autologous CAR-T therapy, and can allow patients who have dysfunctional T cells, due to prior treatments, to receive therapy.
While allogeneic CAR-Ts address logistical challenges associated with autologous CAR-Ts, they come with an increased risk of graft versus host disease (GvHD), which is a result of the body rejecting the newly introduced foreign T cells. Cytokine Release Syndrome (CRS) is the largest safety concern for cell therapy and is a condition that may occur after treatment with some types of immunotherapy. Allogeneic T cell therapy does not show an increased risk of CRS when compared to CAR-T cell therapy, however more data is required to conclude this. If so, allogenic T cell therapy could have a considerable advantage against traditional CAR-T cell therapy.
T Cell Receptor (TCR) T Cells
T Cell Receptor (TCR) T cells recognize and bind to tumor-specific proteins and kill cancerous cells.
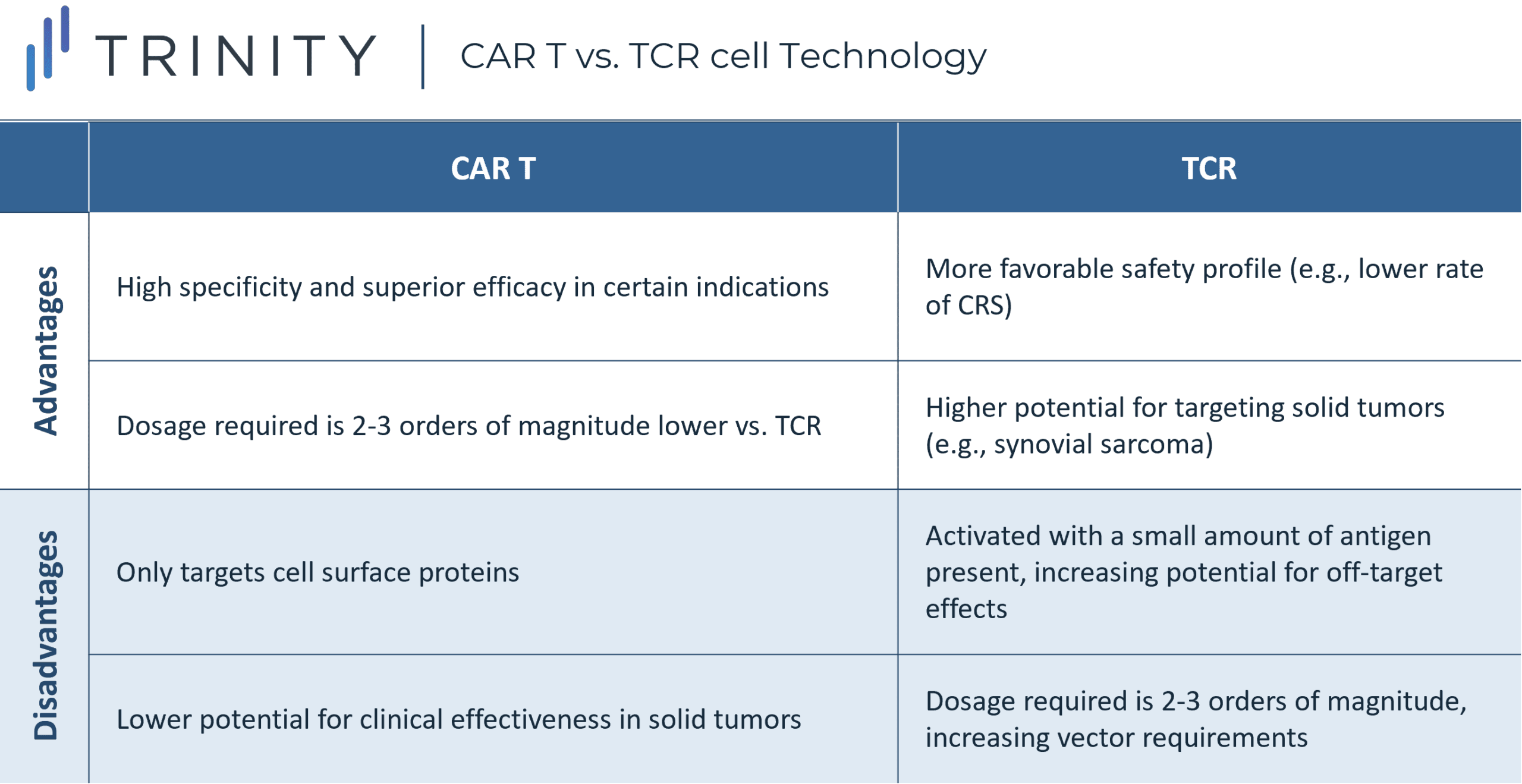
The complementarity determining regions of T cells are modified to enhance the T cell’s affinity to the cancer cell’s human leukocyte antigen (HLA) peptide complex. This mechanism differs from CAR-T therapies, which recognize cell surface proteins only. Since TCR T cells must match the appropriate HLA restriction element to present tumor reactivity, the HLA profile of patients must be determined through lab testing.
Although the wider range of protein targets presents an advantage for TCR T cell therapy, this approach is dependent on the presentation of major histocompatibility complex (MHC) molecules, to recognize the target protein or antigen. This means that malfunctioning cells may escape immune surveillance if the MHC protein is downregulated or mutated. Additionally, the dosage of CAR-T cell therapy is two to three orders of magnitude lower than the required dosage needed for TCR T cell therapy. This is because CAR-T cells do not have an MHC restriction but have a clear tumor surface target and act with high specificity.
TCR T cell technology development has various challenges including selecting appropriate target antigens, searching for specific TCRs, and finding the optimal TCR affinity. Furthermore, there is a risk of the modified TCR T cell’s target not being the intended target, which may induce harmful recognition of autoantigens and lead to GvHD.
The safety profile of TCR T cell therapies has been demonstrated to be more tolerable when compared to CAR-Ts, given the lower rates of CRS. TCR T cell therapies are in development for several different solid tumor types, including metastatic melanoma, synovial cell sarcoma, multiple myeloma, and esophageal cancer, given their ability to target proteins unique to specific tumors driven by intracellular protein recognition. Leading companies in this space include: Adaptimmune, Medigene, and KitePharma.
Non-T cell Therapies
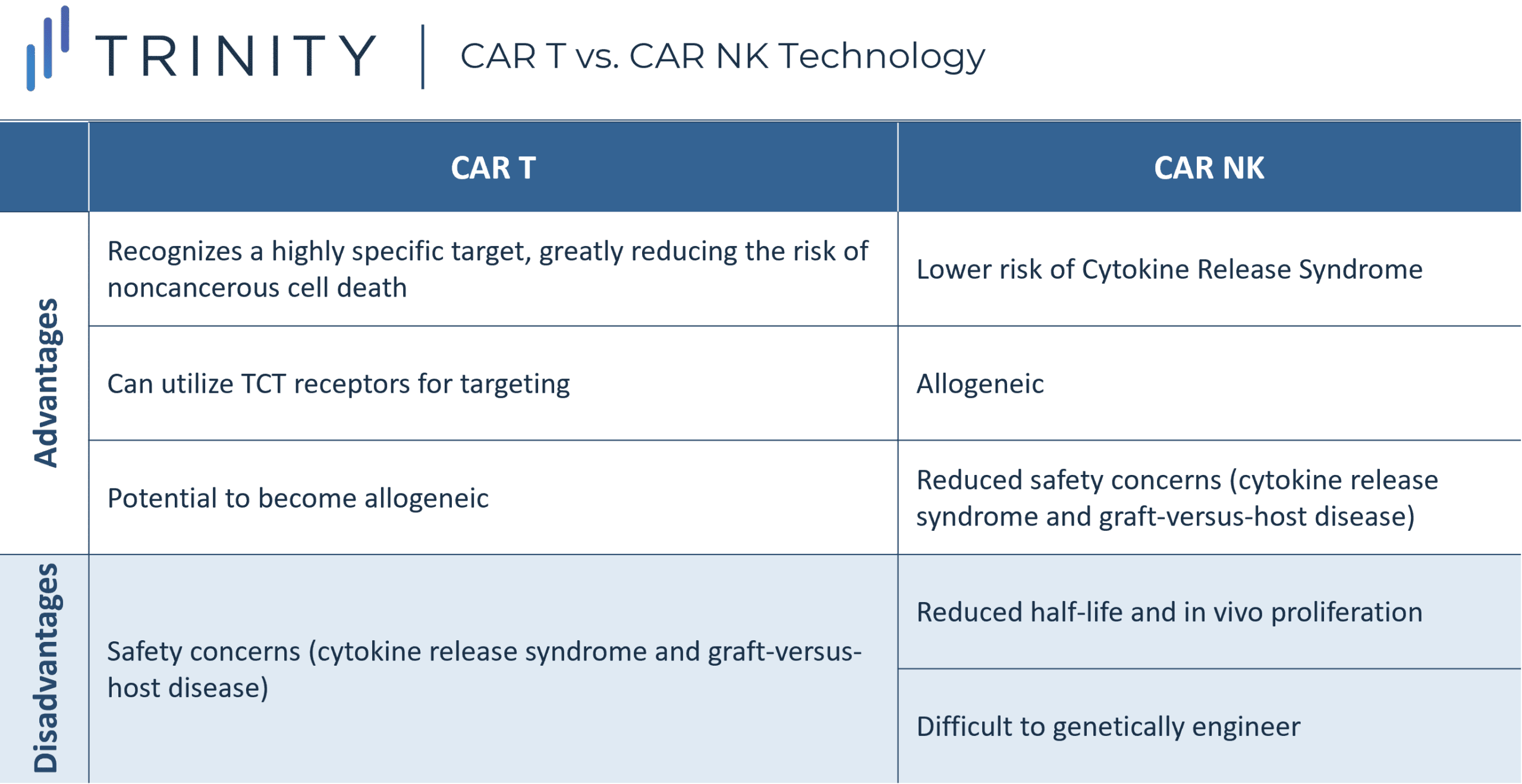
CAR technology is being applied to other immune cells to fight cancer, with significant interest in Natural Killer (NK) cells. NK cells are part of the innate immune response, rapidly kill cells that are ‘non-self.’ Therefore, they should be ideal for treating cancer with broad spontaneous cytotoxic activity without the need for specific antigen recognition.
Unlike T cells, NK cells do not require strict human leukocyte antigen (HLA) matching, eliminating the risk of GvHD. This means NK cells are naturally allogeneic and would be available as off-the-shelf products. In addition, NK cells have a decreased likelihood of causing CRS given they release different cytokines vs. T cells (that mainly produce proinflammatory cytokines) and NK cells do not undergo clonal expansion.
Research into NKs is gaining momentum with significant investment by new biotech companies recently. The most advanced asset is TAK-007, an off-the-shelf CAR NK cell therapy developed by Takeda in partnership with MD Anderson. This therapy may treat B Lymphoid Malignancies through an engineered CAR receptor targeting the CD19 antigen. While obstacles such as transfection and expansion techniques are being overcome, future work will focus on increasing the life-span of CAR NK cells to ensure long-term surveillance, so that patients do not need to be re-treated, creating risk of cancer resistance.
The activity of CAR NK cells in solid tumors is also being investigated; however, these therapies face similar challenges to CAR-Ts in finding the right CAR antigen target and dealing with the immunosuppressive microenvironment. Another alternative to CAR-Ts is CAR-carrying macrophages (CAR-M). Carisma Therapeutics is the leader in this technology and is in pre-clinical development. Earlier this year they published proof-of concept work in Nature Biotechnology, and the FDA cleared the investigational new drug application for their asset, CT-0508, which targets solid tumors that overexpress HER2.
Combination Therapies
An alternative strategy being explored is to administer CAR-T in combination with an immune checkpoint inhibitor. The rationale being cancerous cells may upregulate checkpoint inhibitors such as PD-1 to evade the immune system, upon T cell activation. Administering an immune checkpoint inhibitor post-CAR-T infusion may enhance and prolong the activity of CAR-T cells.
The phase I/II ZUMA-6 trial ran by Gilead Sciences tested the feasibility of this approach by combining its YESCARTA with Roche’s PD-L1 inhibitor TECENTRIQ in DLBCL. Results showed a manageable safety profile with efficacy outcomes and CAR-T cell levels similar to those of patients treated with YESCARTA alone. Despite the lack of increased efficacy demonstrated in DLBCL, CAR-T / immune checkpoint inhibitor combinations may show potential in the solid tumor space. To-date, cell therapies have shown limited efficacy in this space. This is primarily due to solid tumors expressing checkpoint inhibitor ligands, that inhibit the activity of CAR-Ts. By administering immune checkpoint inhibitors, these evasion measures can be neutralized, allowing for CAR-Ts to target and kill the cancerous cells.
If access and reimbursement challenges can be overcome, we can envision a future of personalized medicine where patients are stratified by biomarkers (e.g., PD-L1, CTLA-4) to receive different targeted biology treatments in combination with cell therapies.
The Future of Cell Therapy for Cancer Treatment and other Therapeutic Areas
Allogeneic CAR-Ts are likely to be the next step forward in cell therapies, providing the potential to reduce logistical complexities associated with current autologous CAR-Ts. Costs are likely to drop as products become ‘off-the-shelf’, and outcomes may improve at the patient-level, if patients do not have to wait for manufacturing. This accessibility may allow allogeneic CAR-Ts to be more broadly available than autologous CAR-Ts are today.
Pivoting from highly specialized individual products to a more traditional and sustainable approach, will be a major step for cell therapies in the short-term, and it is likely to be perceived positively by payers and healthcare practitioners, so long as safety risks such as graft-versus-host disease can be overcome.
Effectively treating solid tumors is likely to be another significant breakthrough for cell-based therapies. With a significant amount of research and innovation focused on alternative cell therapy approaches, such as TCR Ts and CAR NKs.
While current cell therapy research is primarily focused on oncology, opportunities exist for cell therapies to provide significant value to patients in other therapeutic areas. The curative potential of cell therapies such as CAR-Ts is being explored in a range of autoimmune and inflammatory diseases (e.g., type 1 diabetes). Additionally, cellular therapies with polyclonal regulatory T-cells (CAR Tregs) may offer potential to provide targeted immunosuppression following organ or hematopoietic stem cell transplantation.
As cell therapy technologies expand to become more accessible, less toxic, and target a wider range of tumor types, early payer and provider engagement will be a critical component of ensuring that these therapies reach their full clinical and commercial potential.
Written by: Sophie Katz, Michaela Broadhurst, William Bolton, and William Rienas