Rise with the Waves: Canada – A Coming (R)evolution: Transforming Pharmaceutical Market Access
Executive Summary
Canada has undertaken meaningful strategies to transform patient access and ultimately improve health outcomes across the country. Two such potentially high-impact strategies include the planned transformation of the former Canadian Agency for Drugs and Technology in Health (CADTH) into the new Canadian Drug Agency (CDA) over the course of the next five years, as well as the implementation of time-limited reimbursement for specific drugs.
While the Canadian Drug Agency is set to advance Canada’s pharmaceutical system by standardizing prescription practices and broadening access to Pan-Canadian health data, time-limited reimbursement is being piloted with the aim to grant prompt access to promising drugs for patients with high unmet need.
Despite both being in their early stages, it is important for pharmaceutical manufactures seeking to enter the Canadian market to monitor the evolution of these policies which are likely to shape Canada’s healthcare system.

This blog highlights the aforementioned high-impact policies recently adopted in Canada, and the opportunities and challenges that pharmaceutical manufacturers will have to consider to optimize the pricing and market access potential for their assets.
Key Policy Updates
The Replacement of CADTH with CDA: Not Just a New Abbreviation
Upon analyzing the extent of inconsistency in prescription standards, overprescription of drugs and lack of health data access in the country over the years, the Government of Canada announced on December 18, 2023 plans for a significant transformation of CADTH into the CDA to address the aforementioned challenges. While it may seemingly appear as a simple name change to a less convoluted title, the CDA is expected to deliver the dedicated leadership and coordination required to make Canada’s drug system more sustainable and efficient in delivering consistent and standardized health outcomes.
The replacement of the CADTH with the CDA is set to materialize over the course of the next five years with an additional investment of $89.5 million CAD on top of the $34.2 million CAD already designated for the annual funding of CADTH.
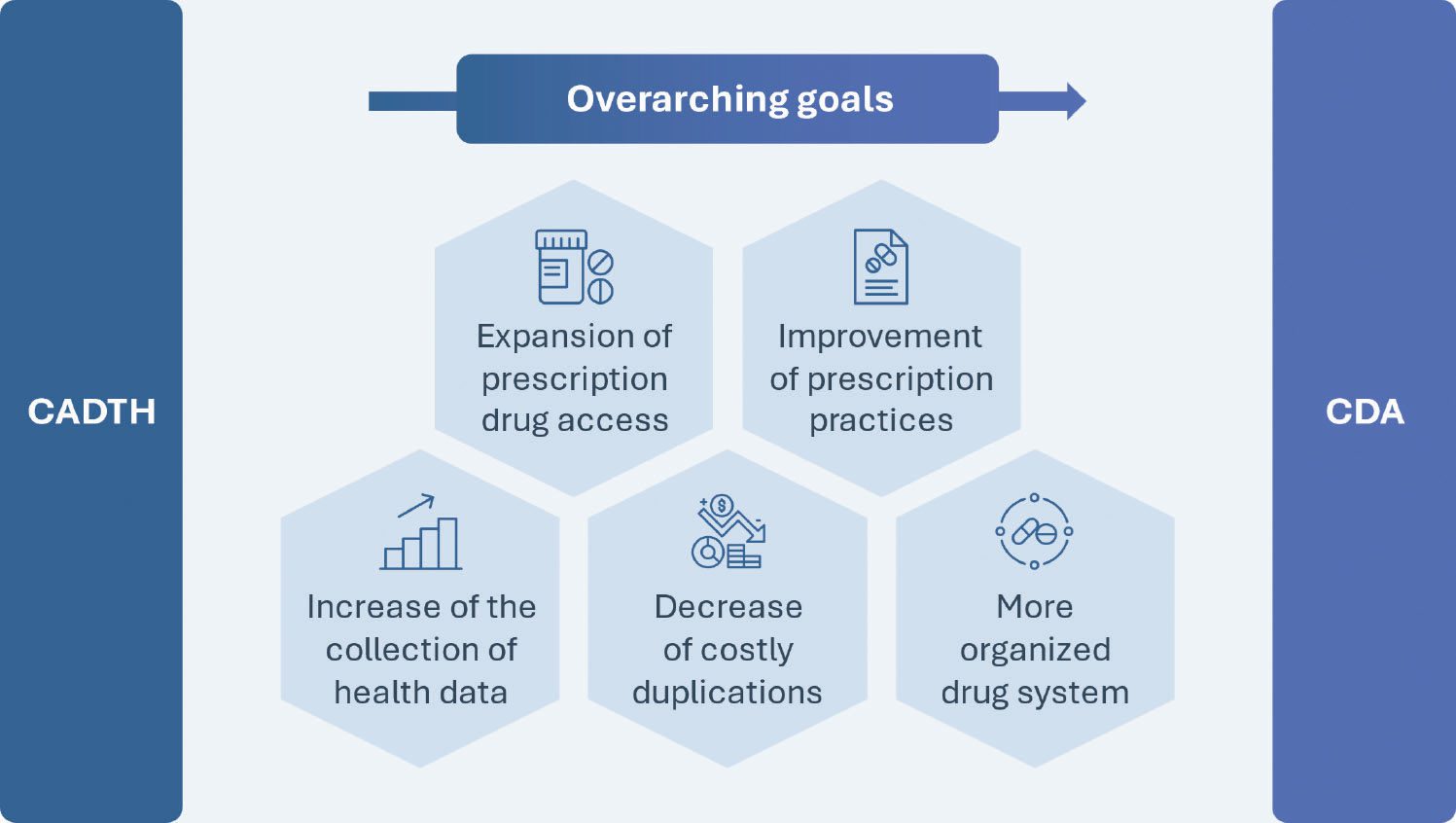
The plans to create the CDA were first unveiled in 2019 with a Canadian Drug Agency Transition Office (CDATO) being established in 2021, setting up collaborations with provinces and territories (PTs) as well as industry experts, patients, providers and indigenous leaders to outline the current key shortcomings of Canada’s pharmaceutical system. Two years later, the CDA was created with the aim of expanding prescription drug access, improving prescription practices, increasing the collection of health data, prioritizing real-world evidence (RWE), and decreasing costly duplications and disorganization within the drug system. As such, beyond addressing the main incompetencies mentioned before, the CDA will also progressively become responsible for health technology assessments (HTAs), post-market safety and effectiveness evaluation, regulating drug prices, negotiating a national formulary and cultivating a universal pharmacare program.
HIGH PRICING AND MARKET ACCESS IMPACT: Monitoring CADTH’s progressive shift to the CDA will be important for manufacturers seeking to launch their assets in Canada, particularly in terms of any associated shifts in current HTA processes, standards and timelines. The CDA is expected to mitigate current affordability and access challenges related to current drug prescribing practices while expanding healthcare data collection including real-world evidence to improve decision-making and optimize drug access. It is also imperative for manufacturers to ensure alignment of their strategies with the transformative policies of the CDA, directed towards the long-term sustainability of Canada’s drug system.
FUTURE OUTLOOK: CADTH’s transformation into the CDA will bring about potential changes in HTA processes while implementing the expansion of data-driven drug access and development of consistent prescribing practice standards. Time will determine if these initiatives will be successful in achieving their intended outcomes. As these changes are implemented, their true impact on Canada’s pharmaceutical landscape will become clearer.
Time-limited Reimbursement Recommendations: Balancing Drug Uncertainties with Patient Need
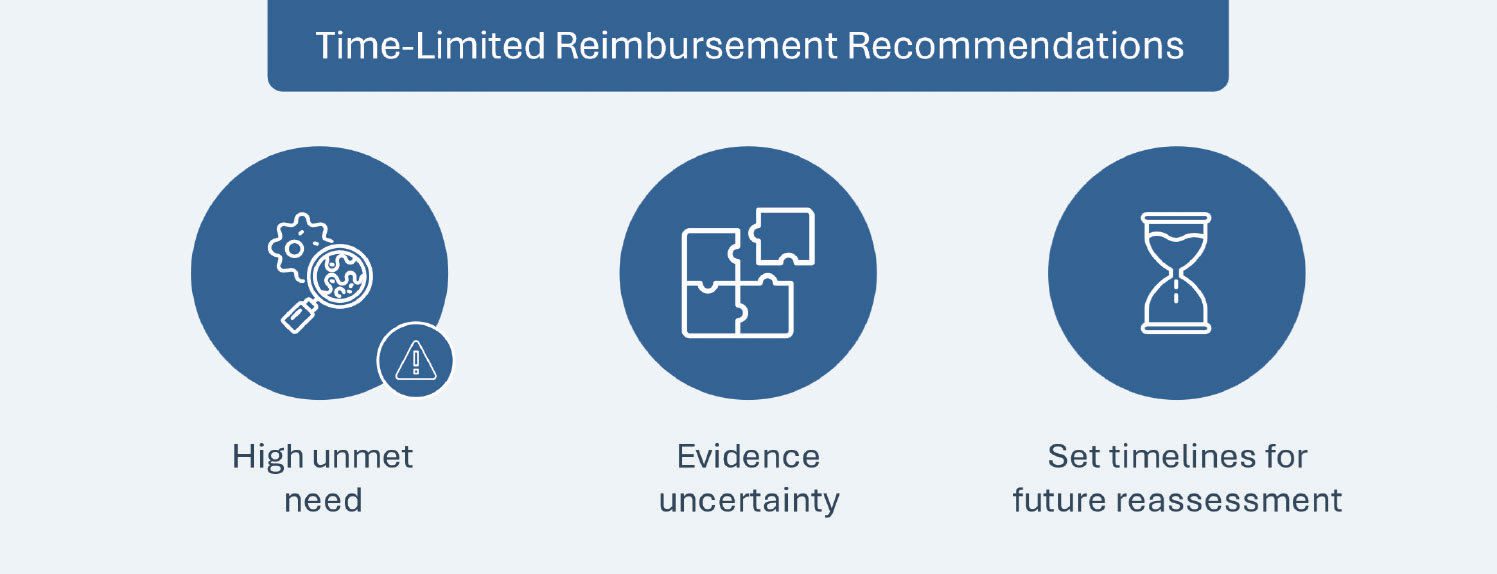
Following in the footsteps of other early access schemes, such as France’s Early Access Authorization or the UK’s Early Access to Medicines Scheme, on September 28, 2023 CADTH introduced time-limited drug reimbursement recommendations for promising new treatments that target the unmet needs of patients living with severe, rare or debilitating illnesses, but have significant evidence gaps in the preliminary clinical studies submitted for CADTH’s review. The recommendation for temporary funding will be provided by CADTH under the condition that the respective manufacturers collect and submit additional evidence (clinical and/or economic) to address the uncertainties identified in CADTH’s assessment. Drugs are only eligible for the recommendation if all the following criteria are satisfied:
- Notice of Compliance with Conditions (NOC/c): A prior or an ongoing assessment under NOC/c policy from Health Canada for conditional drug marketing (undertake further studies to verify the drug’s clinical benefit(s)) with Phase III studies
- Evidence-generation plans: Phase III study plans aligned with the patient population in the indication submitted to CADTH (e.g., same line of therapy and same dosing regimen) and a target completion time of three years from the application
- Evidentiary gaps: Validation that evidence generation plans are set to address the uncertainties described by CADTH’s expert committee
- Manufacturer’s commitment: The manufacturer commits to filing a future reassessment application of its additional evidence once the new evidence has been generated. CADTH will then provide a final reimbursement recommendation
For all the drugs following the time-limited reimbursement pathway, a temporary risk-sharing agreement will thus be negotiated between manufacturers and payers, which will require pricing of the respective products in question according to established cost-effectiveness per the offset needed for the clinical uncertainty associated with its evidence package.
Additionally, CADTH notes successful time-limited reimbursement recommendations and reassessment outcomes will require manufacturers to enhance engagement efforts to ensure optimal launch strategies, fulfillment and feasibility management of evidence requirements, as well as evaluation of risk vs. benefit of temporary access.
HIGH PRICING AND MARKET ACCESS IMPACT: CADTH’s time-limited reimbursement scheme has been implemented to reduce access barriers for patients with a high unmet need triggered by the existence of data uncertainties while still accounting for key unknown factors needed to conduct a comprehensive assessment of applicable treatments. Additionally, the eligibility criteria set forth by CADTH are expected to standardize reassessment procedures and expectations for manufacturers since failure to submit the requisite data within the pre-determined timelines will result in deprivation of funding for such treatments.
FUTURE OUTLOOK: Manufacturers must weigh any trade-offs between temporary access via time-limited reimbursement and the required risk-sharing agreement proposals and commitment to timely reassessment to offset clinical uncertainty. The time-limited reimbursement recommendation process may eventually be expanded to cover other types of drugs. CADTH is expected to evaluate the process after the initial set of recommendations (three to five) have been issued, or after 18 months, whichever is sooner. This pathway will remain available during this evaluation period.
Conclusions
Recent and ongoing policy updates in Canada such as the ones discussed herein clearly reflect the country’s emphasis on reducing access barriers while optimizing patient access to drugs. Whether indirectly or directly, Canada’s efforts to optimize access also highlight the opportunity for improved commercial potential for manufacturers.
Manufacturers can take advantage of CADTH’s shift to the CDA by closely monitoring the developments and opportunities for engagement to target speedier time-to-access in this market. Furthermore, manufacturers can strategically leverage the new time-limited reimbursements by identifying eligible assets and closely following qualifying criteria. Time-limited recommendations will enable earlier access to new therapies indicated for severe, rare or debilitating conditions where there is an unmet medical need. It is imperative for manufacturers to identify the eligibility criteria for their assets and accordingly, revisit clinical and economic evidence for their assets.
If manufacturers can adhere to these policies, both time-limited reimbursements and the development of the CDA have the potential to significantly impact pricing and market access of drugs across Canada.
Further Impactful Policy Changes
UPCOMING: The Patented Medicine Prices Review Board (PMPRB) is reviewing feedback to the proposed approach for reference-based pricing and is set to announce next steps and publish a separate report with analyses of the submitted feedback in the near future.
AUTHORS: Karla Sanchez Gonzalez, Lily Keiderling, Andreia Ribeiro, Nikhil Taxak, Harshmani Sapra, Salome Monreal, Maximilan Hunt